| Phone: |
+420 54949 3805, +420 54949 8166 |
| E-mail: |
|
| Office: |
|
Research areas
-
Carbohydrate-binding proteins involved in host-pathogen interactions
-
Human glycosyltransferases and their role in cancerogenesis
-
Glycosyltransferases participating in mycobacterial cell wall synthesis
-
Protein engineering of lectins
-
Biomolecular interactions
Main objectives
-
To study the therapeutical aspects of recognition and adhesion phenomena in host-pathogen interactions.
-
Investigations of the structures and interactions of biomacromolecules and their relations to the functions of living systems, disease and therapy.
Content of research
Research focusses on structure-function studies of proteins which participate in oligo-saccharide syntheses (glycosyltransferases), or specific recognition (lectins). Carbohydrates are essential for life and, in addition to their conventional role as structural and energy storage components, they play crucial roles in many recognition events. Specific recognition of glycoconjugates is an important event in biological systems and participates in numerous physiological and pathophysiological processes including cell signalling, differentiation, fertilization, infl ammatory response, as well as in cancerogenesis or pathogen-cell adhesion and recognition. The multiple roles of carbohydrates arise from the stability and the stereochemical diversity of glycosidic bonds.
The life cycles of pathogenic bacteria, fungi and viruses require the specific recognition of host tissue for adhesion and subsequent invasion. A common strategy often used by pathogens involves binding to the host glycoconjugates through sugar-binding proteins that can display exquisite specificity for the target tissue. For many pathogenic organisms, the ability to adhere to host tissues is essential to initiate an infection. Oligosaccharide-mediated recognition and adhesion are, for example, key points in the early steps of P. aeruginosa infection because they are followed by processes such as chronic colonization and biofilm formation together with alginate production under the control of quorum sensing. These last adaptive changes by the bacteria make their eradication difficult because, when in the biofilm phenotype, the bacteria become resistant to antibiotic drugs. This brings new challenges into the therapeutical area because the inhibition of protein-carbohydrate interaction could lead to the inhibition of pathogen recognition and adhesion to the host cell.
One of the groups of proteins we are interested in is a group of lectins from human opportunistic pathogens, such as Pseudomonas aeruginosa, Burkholderia cepacia complex, Aspergillus fumigatus, etc. These organisms are usually widely spread around the population and are not dangerous to healthy individuals. On the other hand, they become highly virulent in contact with immunocompromised patients. Infections developed are associated with high morbidity and mortality in the infected people, especially in individuals under mechanical ventilation and in patients suffering from cystic fibrosis.
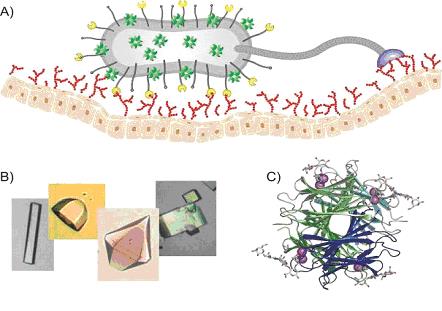
Carbohydrate-binding proteins
A) Schematic representation of carbohydrate-binding proteins at the top of pili and flagella responsible for the attachment of the pathogenic bacteria to the epitelial host cell carbohydrates.
B) Crystals of several protein/carbohydrate complexes allowing their structure determination using X-ray.
C) X-ray structure of PA-IIL lectin from Pseudomonas aeruginosa complexed with sugar epitope LNnFP-V (PDB 1W8F).
We use a multidisciplinary approach to study the recognition and adhesion phenomena of pathogens including bacteria, viruses, and fungi to human tissue. We have been identifying new potential protein targets using bioinformatics tools, and they have been prepared in recombinant form using molecular biology approaches. The complementary techniques of binding experiments, isothermal titration microcalorimetry, surface plasmon resonance and high resolution X-ray crystallography are used to decipher the thermodynamic and structural basis for the unusually high-affinity binding of lectins from pathogens to their host carbohydrates. In addition, site-directed mutagenesis in combination with structural and functional studies allow us to understand the roles of particular amino acids in the fine definition of sugar specificity and preference.
Such a structure-function correlation of protein/carbohydrate interaction forms the basis for the rational design of carbohydrate-based drugs directed against adhesion and virulence.
CURRENT RESEARCH INFRASTRUCTURE
The current infrastructure includes SPR Biacore 3000, isothermal titration calorimeters, AutoITC200, VP-DSC differential scanning calorimeter, DynaPro Plate Reader dynamic light scattering plate reader, Jasco 850 CD spectrophotometer, ProteomLab XL-1 analytical ultracentrifuge, Mosquito crystallization robot, Rigaku Desktop Minstrel UV + Plate Hotel Gallery 160 automatic visualization and documentation system for crystallization, Tecan Evo150 automatic pipetting station, PM-1s automatic colony picker, AKTA Purifier, AKTA FPLC, common equipment for molecular biology and biochemistry work.
1. Structure-functional study of proteins involved in host cell recognition
Supervisor: prof. RNDr. Michaela Wimmerová, Ph.D.
Annotation
Lectins are ubiquitous carbohydrate-binding proteins, which play a key role in various processes including cell-cell communication and host-pathogen interaction, but also serve as a valuable tool for medicine and life sciences research. Carbohydrate-mediated recognition plays an important role in the ability of pathogenic bacteria to adhere to the surface of the host cell in the first step of their invasion and infectivity. Lectin-carbohydrate interactions are usually characterised by a low affinity for monovalent ligands that is balanced by multivalency resulting in high avidity for complex glycans or cell surfaces.
The main aim of the PhD work will be the structure-functional studies of carbohydrate binding proteins involved in a bacterial pathogenesis and/or their application as the bioanalytical tool to study a specific glycosylation related to cell specific tissues.
Keywords: lectins, pathogens, X-ray crystallography, biomolecular interactions, tissue specific glycosylation