| Phone: |
+420 54949 6876 |
| E-mail: |
,
|
| Office: |
|
Research areas
-
Biology of the non-coding RNAs (microRNA, T-UCR, LncRNA, pyknons, etc.) and their involvement in carcinogenesis
-
Significance of non-coding RNAs in solid cancer pathogenesis and identification of new therapeutic targets
-
Application of non-coding RNAs in solid cancer diagnostics and individualization of therapy in cancer patients
Main objectives
-
Introduction of high-throughput analyses (whole genome sequencing and transcriptome profiling) of human genome mainly focused on non-coding RNAs. Utilization of these technologies in medicine and development of diagnostic tests based on high-throughput methods.
-
Comprehensive analysis of non-coding RNAs (expression, SNPs, methylation profiles, according to Objective 1) in solid cancer (mainly colorectal cancer, renal cell carcinoma, esophageal cancer, breast cancer, lung cancer and glioblastoma multiforme). Integration of experimental data with clinico-pathological characteristics of patients aiming identification of potential susceptibility, diagnostic, prognostic, and predictive biomarkers, as well as new therapeutic targets in tumor tissue or patient’s body fluids. Design and coordination of large multi-centric validation studies.
-
Detailed phenotypic characterization (e.g. validation of predicted targets of miRNAs and their integration into signalling pathways) and functional evaluation (proliferation, cell cycle, apoptosis, invasiveness, etc.) of non-coding RNAs suspected to be involved in carcinogenesis or cancer outcome (Objective 2) in vitro in a relevant cell line models. Studies examining oncogenic or tumor-suppressive function of particular non-coding RNA in vivo, with subsequent pharmacological analysis evaluating usage of this RNA as therapeutic target.
-
Formulation and design of recommendations for potential implementation of novel biomarkers (according to Objectives 2 and 3) to clinical management of solid cancer patients leading to higher level of individualization and better therapeutic outcomes. Development and technological transfer of new targeted therapeutic strategies in solid cancer.
Content of research
According to the central dogma of molecular biology, it has been postulated that vast majority of genetic information encoding the biologic form and phenotype is rendered by proteins. Employing whole-genome analytical approaches and next-generation sequencing technologies (ENCODE Project), it has been observed that at least 90% of the human genome is actively transcribed. Although initially argued to be spurious transcriptional noise or accumulated evolutionary debris arising from the early assembly of genes and/or the insertion of mobile genetic elements, recent evidence suggests that such a “dark matter” of the genome, i.e. the non-coding RNAs (ncRNAs) may play the major biological roles in cellular development, physiology and pathologies.
NcRNAs could be grouped into two major classes – small ncRNAs and long ncRNAs. LncRNA are transcripts sized in a range of 200 nt up to 100 kb lacking open reading frames. LncRNA expression levels appear to be lower than protein-coding genes, and some lncRNAs are preferentially expressed in specific tissues. The small number of characterized human lncRNAs have been associated with a spectrum of biological processes, for example, epigenetics, alternative splicing, nuclear import, as structural components, as precursors to small RNAs and even as regulators of mRNA decay. Furthermore, accumulating reports of mis-regulated lncRNA (HOTAIR, MALAT1, HULC, T-UCRs, etc.) expressions across numerous cancer types suggest that aberrant lncRNA expression may be an important contributor to tumorigenesis. Small ncRNAs are represented by a broad range of known and newly discovered RNA species, with many being associated with 5’ or 3’ regions of coding genes. This class includes the well-documented miRNAs, and siRNAs, and most of them significantly extended the concept of molecular carcinogenesis, and recently are subject of the intensive translational research in this field.
Based on commonly accepted definition by Hanahan and Weinberg, the tumor is characterized by 6 features: self-sufficiency in growth signals, insensitivity to growth inhibitory signals, evasion of apoptosis, limitless replicative potential, sustained angiogenesis and tissue invasion and metastasis. It has been repeatedly confirmed that miRNAs may modify each of these six characteristics by multilevel targeting either oncogenes or tumor suppressors. For details see Vogelstein model of carcinogenesis in colorectal cancer.
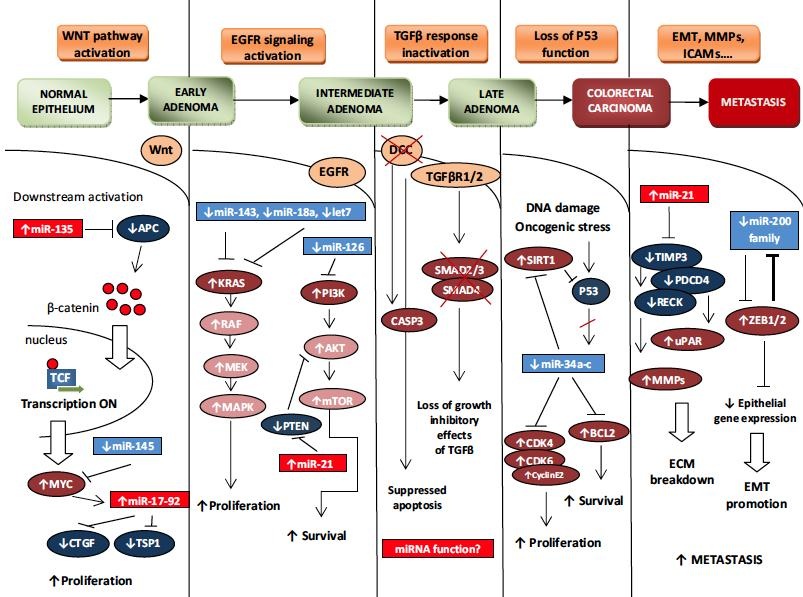
In our research we are mainly focused on colorectal cancer (CRC), significance of ncRNAs in its pathogenesis, and potential utilization of ncRNAs as biomarkers or even therapeutic targets. One of our projects is aiming at new molecular markers enabling the response prediction to anti-EGFR therapy in patients with metastatic CRC (mCRC) with wild type KRAS. Such markers could possibly lead to better understanding of molecular mechanisms of resistance, but also to individualization of therapy and therefore achievement of better therapeutical results and higher quality of life in patients with metastatic colorectal cancer. Furthermore, we are focused on identification of new biomarkers in blood serum. The rationale of our project is to build diagnostic panel of microRNAs enabling early detection of CRC in asymptomatic patients for screening purposes or monitoring of CRC patients with hereditary syndroms, as well as sensitive detection of disease progression in follow-up of CRC patients. We study circulating miRNAs as potential biomarkers also in renal cell carcinoma and pancreatic cancer. Moreover, we are looking for miRNA based diagnostic panel which will enable improved stratification of patients with Barrett esophageus (BE) to subgroups according to their individual risk of progression to esophageal adenocarcinoma, particularly it will enable the „high-risk“ subgroups detection. More accurate risk prediction in BE patients enable more rationalized monitoring design and early detection of progression to higher dysplastic grade or EAC. Another field of interest of our research group is concentrated on study of miRNAs and genes associated with process of epithelial-to-mesenchymal transition. These miRNAs and genes could serve as potential markers of risk prediction and early detection of metastatic disease in renal cell carcinoma patients. Regarding glioblastoma multiforme (GBM), the main goal of our project is to extend knowledge about molecular mechanisms involved in glioblastoma carcinogenesis and invasivness by analysis of miRNA expression profiles in tumors and use our findings for prediction of therapy response in patients with glioblastoma. Furthermore, we would like to contribute to the molecular characterization of glioblastoma stem cells (GSCs) which are involved in resistance to the therapy and relapse of GBM. In this project, miRNA expression profiles of GSCs and non-GSCs will be identified, and furthermore, the obtained data will be used for development of the predictive miRNA panel that will enable prediction of therapy response and prognosis, and development of new, miRNA-based therapies in GBM.
list / cards

Research Group Leader

Researcher

Specialist

PhD student

Researcher

docent, přednosta kliniky

odborný asistent

PhD student

senior researcher

Researcher

PhD student

odborná pracovnice - PhD student

odborný pracovník - PhD student

PhD student

odborná pracovnice

Researcher

PhD student

odborná pracovnice

odborná pracovnice

odborný asistent

odborná pracovnice ve výzkumu - postdoc

laborantka

Pregraduate student

senior researcher

chovatelka laboratorních zvířat