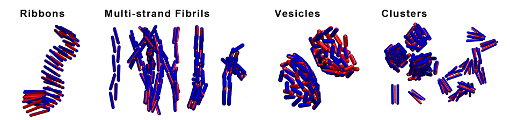
Self-assembly of peptides in solution
Proteins can aggregate in a wide variety of structures, which can be used to spontaneous formation of new peptide based materials. We study self-assembly of a simple model of a rod like particle with attractive (hydrophobic) stripe on its side. All predicted structures by our model were identified with an experimentally observed protein self assembled structures including Coiled Coils, amyloid Ribbons, barrels, and vesicles. Our Monte Carlo simulations showed that aggregate morphologies crucially depend on two parameters. The first one is the width of the attractive stripe and the second one is a presence or absence of attractive interactions at the particle ends. The results provide us a generic insight into conditions needed for a given structure formation and also suggest that other structures that were not observed require more complex model. The model was then modified to two site model by which nucleation and growth of amyloid fibres was studied. The first state corresponds to solution conformations, while the second state represents conformations in fibril. We made a video from our simulation on amyloid formation. [Vácha 2011a, Bieler 2012, Shi 2013, Vácha 2014a]
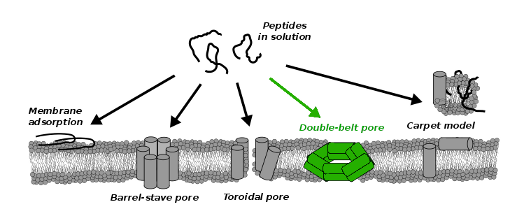
Interaction of peptides with membranes
In living organisms, a enormous number of peptides are interacting with phospholipid membranes, which can have a significant effect on the membrane barrier function. High permeabilization or complete disruption of the protective membrane can result in detrimental cell necrosis. The lethal function is exploited by antimicrobial peptides, which are part of the defense system in many organisms. Antimicrobial peptides kill host bacteria by permeabilization of its membrane, while they are leaving other cells intact, which is why antimicrobial peptides could be used as new antibiotics. Very recent work demonstrates that there is a similarity between antimicrobial and amyloid forming peptides that have been associated with various neurodegenerative diseases such as Parkinson's disease, Alzheimer's disease, and type-II diabetes mellitus. We investigate conditions under which amphiphilic peptides disrupt phospholipid membrane and characterize the disrupting structures by means of computer simulations. Employing coarse grained models we identify crucial parameters of permeabilizing peptides. Our simulations are able to reproduce the formation of the well-known barrel-stave or toroidal pores but, in addition, we find evidence for a novel double-belt pore structure. In this structure the peptides that coat the membrane pore are oriented parallel to the membrane plane. See video from our simulation, where double-belt pore form spontaneously. [Vácha 2014b]
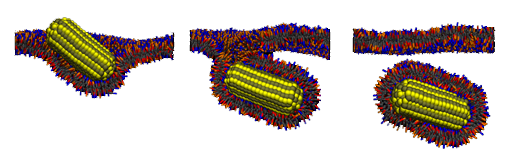
Interaction of nanopartticles with membranes
We investigate conditions of cellular uptake of ligand-coated nanoparticles via passive endocytosis. Our simulations show that when nanoparticles are large enough the uptake across a receptor-rich, phospholipid membrane can be spontaneous (thermodynamically driven) process for both spherical and spherocylindrical shape nanoparticles. However, a presence of sharp edges on nanoparticle could prevent such uptake. These findings are supported by experimental evidence on internalization of silica spheres into lipid vesicles and by our analysis of elastic model. Moreover, our simulations suggest that the spherocylinders could be more efficient in drug delivery, since they seemed to have the same kinetic barrier but larger volume than spheres. We also investigate a pathway by which uptaken nanoparticles can shed the membrane coating. Nanoparticle can escape upon a reduction of ligand-receptor interaction (e.g. achieved by a change of pH) only if the nanoparticle is either very small or nonspherical. In case of large spherical nanoparticle, the reduction of attraction needs to be accompanied by exerting an additional tension on the membrane (e.g., via nanoparticle expansion) to achieve the release. Note that our nanoparticle model can represent virus capsid as well as artificial nanoparticles and as such it reveals crucial ingredients for passive cellular uptake important in nanomedicine. See video of trajectory from our simulations. [Vácha 2011b, Vácha 2012]
PUBLICATIONS
Bieler, N.S.; Knowles, T.P.J.; Frenkel, D.; Vácha, R.; 2012: Connecting Macroscopic Observables and Microscopic Assembly Events in Amyloid Formation Using Coarse Grained Simulations. PLoS Computational Biology, 8 (10), e1002692. doi:10.1371/journal.pcbi.1002692
Shi, Q.; Bergquist, K.-E.; Huo, R.; Li, J.; Lund, M.; Vácha, R.; Sundin, A.; Butkus, E.; Orentas, E.; Warnmark, K.; 2013: Composition- and Size-Controlled Cyclic Self-Assembly by Solvent and C60-Responsive Self-Sorting. Journal of American Chemical Society, 135 (40), 15263-15268. doi:10.1021/ja408582w
Vácha, R.; Linse, S.; Lund, M.; 2014a: Surface Effects on Aggregation Kinetics of Amyloidogenic Peptides. Journal of American Chemical Society. doi:10.1021/ja505502e
Vácha, R.; Frenkel, D.; 2014b: Simulations Suggest Possible Novel Membrane Pore Structure. Langmuir, 30 (5), 1304-1310. doi:10.1021/la402727a
Vácha, R.; Martinez-Veracoechea, F.J.; Frenkel, D.; 2012: Intracellular Release of Endocytosed Nanoparticles Upon a Change of Ligand-Receptor Interaction. ACS Nano, 6 (12), 10598-10605. doi:10.1021/nn303508c
Vácha, R.; Frenkel, D.; 2011a: Relation between Molecular Shape and the Morphology of Self-Assembling Aggregates: A Simulation Study. Biophysical Journal, 101, 1432-1439. doi:10.1016/j.bpj.2011.07.046
Vácha, R.; Martinez-Veracoechea, F.J.; Frenkel, D.; 2011b: Receptor-Mediated Endocytosis of Nanoparticles of Various Shapes. Nano Letters, 11 (12), 5391–5395. doi:10.1021/nl2030213