| Phone: |
+420 54949 7659, +420 54949 7010 |
| E-mail: |
,
|
| Office: |
|
| Phone: |
+420 54949 5606 |
| E-mail: |
,
|
| Office: |
|
Research areas
-
Imaging of biomolecules, cells and other biological objects using scanning probe microscopies (SPM – including AFM, SNOM and STM)
-
Characterization of affinity interactions using biosensors in real time
-
Development of biosensors using electrochemical, optical and piezoelectric transducers
-
Immobilization, modification and conjugation of biomolecules
Main objectives
-
Visualization and modification of biological objects including tissues, cells, cellular structures, and single biomolecules
-
Development of new methodologies for investigating the structure, interactions, and dynamics of biomolecules.
-
Investigations into the structure and interactions of biomacromolecules and their relation to the functions of living systems, disease and therapy.
-
Investigations into the behaviour of natural and chemically modified biomacromolecules at electrically charged surfaces linked to the development of novel electrochemical biosensors and bioassays.
Content of research
Nanobiotechnology represents advanced scanning probe microscopic techniques, nano-lithographic machining and various types of artificial nanostructures applied for either visualization or modification of biological objects including tissues, cells, cellular structures and biomolecules. The unique opportunity to touch a single individual molecule of a protein or nucleic acid with the scanning tip provides high-resolution sub-nanometer and pseudo-3-dimensional images providing details of such bioobjects in their native state. These approaches are currently revolutionizing many fields of biology, biophysics and biochemistry and provide innovative results and methodologies for applications in health care – nanobiosensing systems, nanoparticles for labelling and smart distribution of drugs (nanomedicine). Within this work package, the following research fields will be addressed:
Imaging of biosurfaces and biomolecules using scanning probe microscopies
The laboratory performs atomic force microscopy (AFM) scans in both dry state and in liquids in contact and non-contact modes. Modified tips allow characterization of surface hydrophobicity, and specific target molecules and cellular surfaces (e.g. tips modified with antibodies) are scanned (spectroscopic techniques) while conductive tips with applied potential will be used for bioelectrochemical studies. Repeated scans will allow the movement and morphologic changes of cells to be studied (VideoAFM). Supplementary information about cells and cellular elements will be provided by scanning near optical field microscopy (SNOM, overcoming the diffraction limit); and scanning tunneling microscopy (STM) will be chosen for atomic resolution.
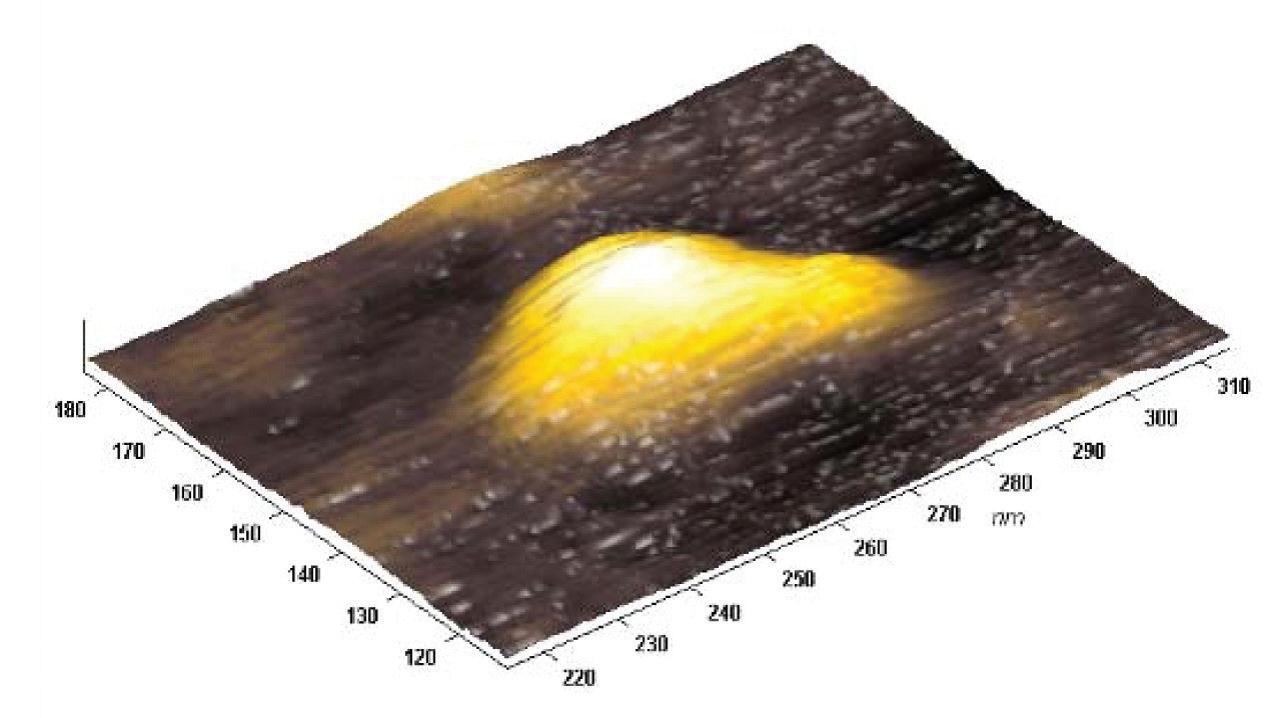
Molecule of immunoglobulin G imaged on the mica surface using the cantilever with a diamond-like carbon tip, semicontact mode, Ntegra Vita atomic force microscope.
Nanobiointeractions and the measurement of forces within biocomplexes
The binding of two individual complementary molecules (antibody-antigen, ligand-receptor, and hybridization of oligonucleotides) will be studied using one partner bound to the solid support and the other linked to the scanning tip. The force data measured at the nanolevel will be correlated with the results obtained at the macrolevel using surface plasmon resonance techniques providing information about kinetics of affinity interactions in real time.
Nanomanipulation and nanolithography of biological objects
In the AFM-lithography mode, the tip of the cantilever in contact with the scanned object can be used to manipulate cells on the surface. Nano(bio)sensing arrays and other functional nanoobjects will be constructed.
Nanobiosensors and biosensing nanoarrays
Nanobiosensors will employ the cantilever as nanomechanical transducer bending due to the affinity interaction on one of its sides. Nanoarrays – biochips consisting of sets of specific recognition proteins (monoclonal or recombinant antibodies, engineered receptors and enzymes, artificial peptide folds designed by molecular modelling) will be incubated with clinical samples and then the binding pattern will be read with the help of AFM (SNOM, STM), either directly or after suitable amplification (magnetic nanoparticles, quantum dots). For the validation, “larger” micrometer-sized array elements will be produced and evaluated using multichannel SPR, fluorescence scanning and scanning electrochemical microscopy (SECM).
CURRENT RESEARCH INFRASTRUCTURE
The infrastructure currently available includes Ntegra Vita AFM, Ntegra Solaris SNOM, Biacore 2000 SPR system, fluorescence microscope, cell cultivation facility, electrochemical (10), piezoelectric (5) and fiber optic (2) detectors, light sources (4), DAD spectrophotometer, Multiskan RC and Synergy 2 plate readers, autoinjectors (2), FIALab 3000, and data acquisition systems (NI). The group members have programming experience in Delphi, C++, Java and LabView.